How Many Electrons Are in Carbon's Valence Shell
The electron configuration of neon shows that the last shell of neon has eight electrons2s 2 2p 6. Two inner shell core electrons in the 1s orbital and four valence.

What Are Valence Electrons Definition And Periodic Table Electrons Electron Configuration Chemical Bond
4 Does GE have 4 valence electrons.

. Oxygen is in group 6 and has 6 valence electrons. Both formally have the same num. It has 6 electrons distributed in 2 energy shells.
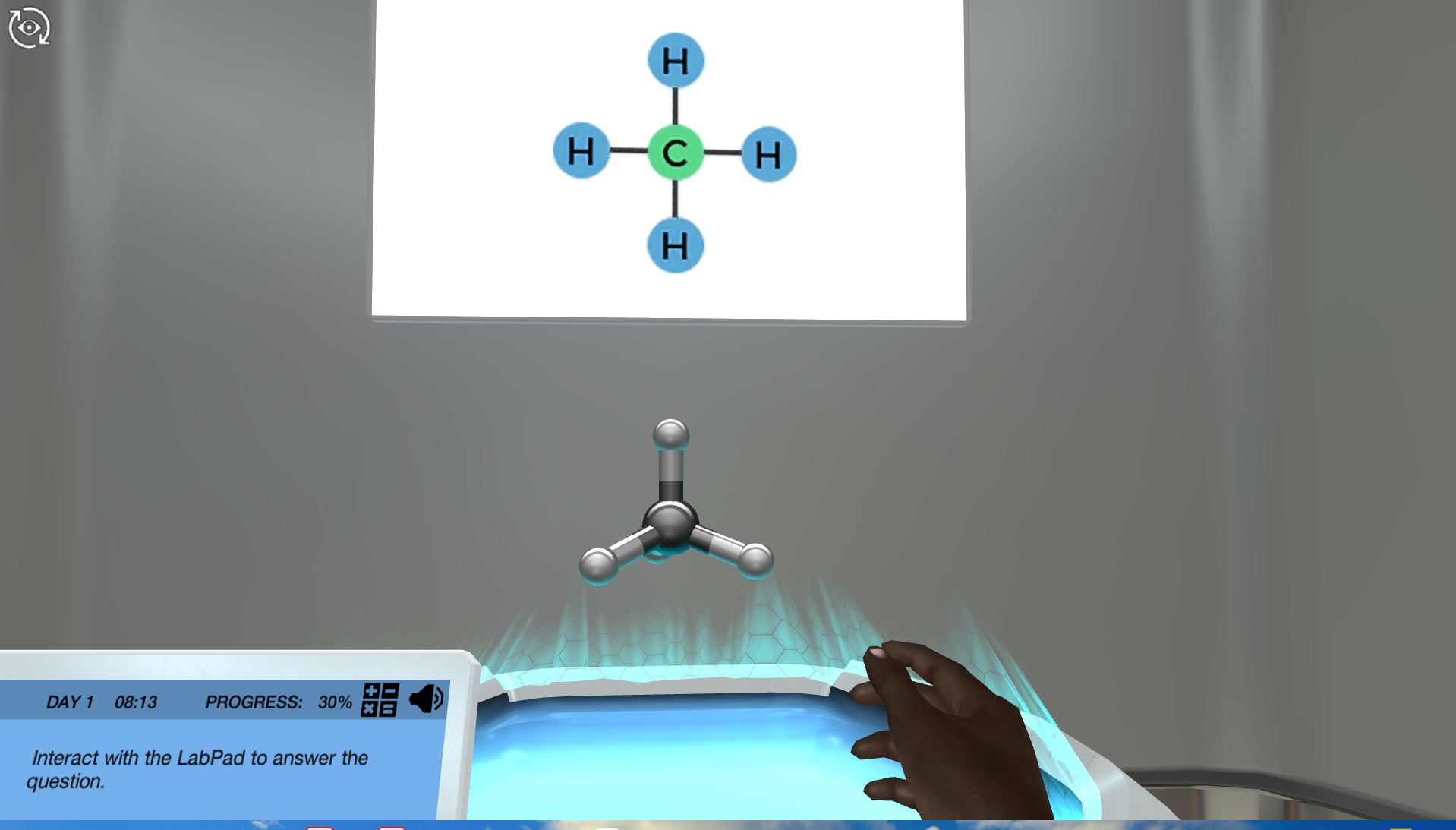
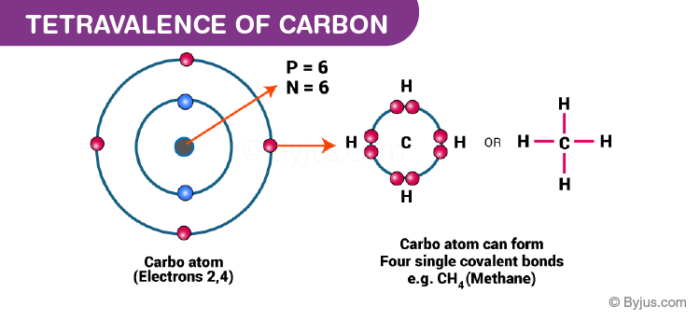
Atomic carbon has six electrons. The sum number of electrons found in an atoms valence shell is referred to as valence electrons and the valence shell of carbon has four electrons 2s22p2. Carbon 4 electrons in the valence shell combines with four hydrogen atoms to form a stable covalent compound where it shares 8 electrons while each hydrogen shares 2.
Electron Configuration for Bonded Carbon When bonded with a full octet such as in methane carbon has eight valence electrons two per covalent bond. The highest energy level of the carbon atom has two subshells. The atomic number of carbon is 6 It is present in group 4 of the Periodic Table of Elements.
Valence electrons in Carbon. Carbon atom has 4 valence electrons. 5 What is the valence shell of GE.
That gives a total of 5 electrons so neutral phosphorus atoms have 5 valence electrons. The last shell after the electron configuration is called the valence shell. There are four valence electrons in carbon as a result of this arrangement.
How many valence shell electrons does carbon atom have. Biology questions and answers. Posted on January 19 2022 By Rishad Hasan Cesium Atomic and Orbital Properties Cesium atoms have 55 electrons and the electronic shell structure is 2 8 18 18 8 1 with Atomic Term Symbol Quantum Numbers 2S12.
6 How do I find valence electrons. Therefore the valence electrons of neonNe are eight. How many valence electrons does Sulfur S Group 16 have.
Here are some examples. The total number of electrons in a valence shell is called a valence electron. Each carbon dioxide molecule is formed from 1 C atom and 2 O atoms.
Two inner shell core electrons in the 1 s orbital and four valence outer most shell electrons in the 2 s and 2 p orbitals. Thus every atom in this stable molecule fulfills the octet rule. 8 Why does C have 4 valence electrons.
How many valence electrons does cesium ion have. 10 How many energy levels and valence electrons does an atom of germanium Ge have. The electron configuration of carbon shows that the last shell of carbon has four electrons2s 2 2p 2.
7 What family has 8 valence electrons. Sulfur has 14 total electrons based on its number of protons and then 6 of these are valence electrons. How many electrons does carbon atomic number 6 contain in its outer valence shell.
Carbon has four empty spaces in its outer shell enabling it to bond to four other atoms. 7 How many electrons does one atom of carbon share to complete its valence shell. There is a point we should pay attention to here.
3 What are the 4 valence electrons. 5 What are the Uses of Carbon. 3 What is Carbon.
For example carbon is in group 4 and has 4 valence electrons. Carbon has 4 valence electrons on the valence shell. To fill the outer shell to 8 electrons an additional 4 are required.
Valence electrons in Carbon is 4. Sulfur has 6 valence electrons because it is in Group 16. The number of valence electrons for molecules can be calculated by adding the valence electrons of all the atoms that form that respective molecule.
Electrons so this level only has two electrons total. 6 How many electrons does a carbon atom have in its outer energy level. Therefore the valence electrons of carbon are four.
How Many Valence Electrons Does Ge Have4 valence electronsHow many valence electrons does GE 2 haveTherefore there are 2 valence electrons in Ge2What are the 4 valence electronsExplanation. The main group number for an element can be found from its column on the periodic table. How many valence electrons does each carbon atom have.
Carbon has a valency of 4 as does every other element in group 4. We know that C has 4 valence electrons and that O has 6 valence electrons which means that the number of. 10 hours agoHelium is located in period 1 group 18 of the Periodic Table and has an atomic number equal to 2.
Therefore there are 2 valence electrons in Ge2. The total number of electrons in a valence shell is called a valence electron. Atomic carbon has six electrons.
Because if you check electron configuration for carbon you will see that last shell is 2s2 2p2 which means that carbon has 4 valence electrons. Helium has 2 electrons in its outer electron shell so 2 dots. 9 How many 4s electrons are in GE.
The highest-numbered shell is the third shell which has 2 electrons in the 3s subshell and 3 electrons in the 3p subshell. Remember dont confuse valence electrons with total electrons. 2 How Many Electrons Can A Carbon Atom Share.
6 b2 O c. Carbon has 4 electrons it its outer shell. 1 How many electrons are in carbon.
It is present in group 4 of the Periodic Table of Elements. 4 What are the Properties of Carbon. Carbon and silicon BOTH come from Group 14 of the Periodic Table ie.
Carbon Valence Hybridization And Angles Virtual Lab

The Number Of Electrons Present In The Valence Shell Of Carbon Of Carbanion Bearing Youtube

How Many Valence Electrons Does Carbon Have Perfect Atom

Carbon C Electron Configuration And Orbital Diagram
Explained How Many Valence Electrons Does Carbon Have Deutschland Herald

Electron Shells Elements 1 18 Youtube

Naming Ions Writing Names Ionic Compound

How Many Electrons Are In Carbon Quora

Mole Relationships Molecular Molecules Relationship

Curious About How Many Valence Electrons Does Carbon Have Norient Beta

Curious About How Many Valence Electrons Does Carbon Have Norient Beta
Valency Of Carbon Tetravalency Hybridization Catenation With Videos

Valence Electrons In Carbon C Youtube

How Many Valence Electrons Does Carbon Have Perfect Atom

Mole Relationships Molecular Molecules Relationship
How Many Valence Electrons Are In A Carbon Atom In The Ground State Quora

Endothermic Lettering System Energy


Comments
Post a Comment